[ad_1]
Washington — A federal judge in Texas on Friday halted the Food and Drug Administration’s approval of the abortion pill mifepristone, delivering a blow to abortion rights advocates in the wake of the Supreme Court’s dismantling of the constitutional right to abortion.
In a 67-page opinion, U.S. District Judge Matthew Kacsmaryk said the FDA’s two-decade-old approval violated a federal rule that allows for accelerated approval for certain drugs and, along with subsequent actions by the agency, was unlawful. He put his decision on hold for seven days to allow for the Biden administration to appeal to the U.S. Court of Appeals for the 5th Circuit.
The Biden administration indeed filed its notice of appeal late Friday night.
In a statement late Friday night strongly condemning the ruling, President Biden said that the “Court in this case has substituted its judgment for FDA, the expert agency that approves drugs. If this ruling were to stand, then there will be virtually no prescription, approved by the FDA, that would be safe from these kinds of political, ideological attacks.”
“The lawsuit, and this ruling, is another unprecedented step in taking away basic freedoms from women and putting their health at risk,” Biden said.
Kacsmaryk’s injunction stopped short of withdrawing or suspending the FDA’s approval of mifepristone, as a group of anti-abortion rights medical associations had asked him to do. Such a move from Kacsmaryk, appointed by former President Donald Trump, likely would have disrupted access to the drug for millions of women nationwide, including in states where abortion is legal.
“The Justice Department strongly disagrees with the decision of the District Court for the Northern District of Texas in Alliance for Hippocratic Medicine v. FDA and will be appealing the court’s decision and seeking a stay pending appeal,” Attorney General Merrick Garland also said in a statement Friday evening. “Today’s decision overturns the FDA’s expert judgment, rendered over two decades ago, that mifepristone is safe and effective.”
But it does further throw into chaos abortion access nationwide amid a legal landscape that has been upended since the Supreme Court reversed Roe v. Wade last June. At least a dozen states have enacted near-total bans on the procedure or imposed tighter restrictions in the wake of the decision to unwind the right to an abortion under the U.S. Constitution.
On the heels of Kacsmaryk’s decision, a federal judge in Washington state issued a decision in a separate case involving mifepristone that preliminarily blocked the Biden administration from altering the status quo as it relates to the availability of the drug. The two competing orders sets up a high-stakes showdown likely to land before the Supreme Court.
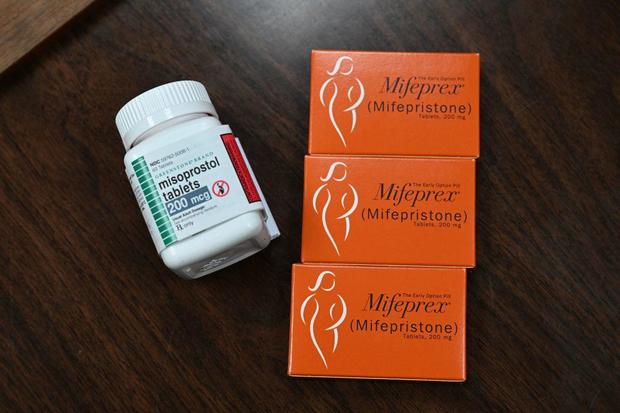
The FDA approved mifepristone more than 20 years ago, and the drug is taken together with a second medicine, misoprostol, to terminate a pregnancy through 10 weeks gestation. Since then, the agency has made several changes to the rules surrounding the abortion pill, including approving a generic version of mifepristone in 2019 and lifting a requirement that the pills be dispensed in-person in 2021, which allowed the drug to be prescribed by a provider during telemedicine appointments and sent by mail.
But it wasn’t until last November — 22 years after the FDA’s approval of the abortion pills — that a group of physicians and medical associations filed a lawsuit seeking to undo the agency’s approval of mifepristone. The lawsuit was filed in the federal district court in Amarillo, where only one judge, Kacsmaryk, is assigned cases.
ROBYN BECK/AFP via Getty Images
Arguments heard in court last month
In their complaint filed by the Alliance Defending Freedom, a conservative legal organization, the doctors argued the FDA erred in determining the abortion drug’s safety and effectiveness and approving it under a federal rule that allows accelerated approval of certain drugs that treat “serious or life-threatening illnesses.” The challengers claim the agency exceeded its regulatory authority to approve the mifepristone and asked the court to issue a preliminary injunction ordering the FDA to undo its approval of mifepristone.
Kacsmaryk held a hearing to consider their request last month.
“The [FDA] must protect the health, safety, and welfare of all Americans by rejecting or limiting the use of dangerous drugs. But the FDA failed America’s women and girls when it approved chemical abortion drugs for use in the United States,” the anti-abortion rights physicians and medical associations told the court. “And it has repeatedly failed them by removing even the most basic precautionary requirements associated with their use.”
But in urging the court to keep the approval of the drug in place, the FDA argued the plaintiffs waited too long to fight its approval of the abortion drug, as challenges to agency actions are subject to a six-year statute of limitations. The agency also noted that while the lawsuit claims the FDA’s approval of mifepristone involved an accelerated review, the drug received the FDA’s OK more than four years after its application from manufacturer Danco Laboratories was submitted.
“Removing access to mifepristone would cause worse health outcomes for patients who rely on the availability of mifepristone to safely and effectively terminate their pregnancies,” the FDA told the court, adding that the “sudden absence” of medication abortion would likely impose “real and significant harms” to patients choosing to take abortion pills out of medical necessity, for privacy or to avoid further trauma.
The FDA also warned that removing the option of medication abortion would lead to overcrowding and delays at clinics already grappling with more patients navigating abortion restrictions in neighboring states.
“This would lead to delays for an array of healthcare services as providers and resources are unnecessarily diverted to surgical abortions,” the agency said.
In his ruling, Kacsmaryk said the groups’ challenges to the FDA’s actions, beginning with its approval in 2000 and to 2021, have a substantial likelihood of success on the merits, and found the agency exceeded its authority in approving mifepristone.
“FDA manipulated and misconstrued the text of Subpart H to greenlight elective chemical abortions on a wide scale,” he wrote, referencing the federal rule under which the abortion pill was approved.
Kacsmaryk also said in his ruling that the “FDA stonewalled judicial review — until now,” and accused the agency of ignoring petitions targeting mifepristone’s approval for 16 years.
“The Court does not second-guess FDA’s decision-making lightly. But here, FDA acquiesced on its legitimate safety concerns — in violation of its statutory duty — based on plainly unsound reasoning and studies that did not support its conclusions,” he wrote. “There is also evidence indicating FDA faced significant political pressure to forego its proposed safety precautions to better advance the political objective of increased ‘access; to chemical abortion — which was the ‘whole idea of mifepristone.'”
But the FDA said its 2000 approval of the abortion pill rested on a “comprehensive evaluation of the scientific data.” The agency explained it reviewed three separate clinical trials involving more than 2,500 pregnant patients and found the trials provided “substantial evidence of effectiveness and showed a low rate of serious adverse events.”
The FDA’s brief also states that additional research involving data on “well over 30,000 patients” shows serious complications associated with mifepristone are rare, involving just a fraction of a percent of cases.
Medication abortions have become more common over the years, accounting for more than half of all abortions in the U.S. in 2020, according to the Centers for Disease Control and Prevention. The American College of Obstetricians and Gynecologists notes medication abortion has been used by over 3 million women in the U.S. since FDA approval in 2000, and says it is “safe and effective.”
Restrictions and challenges to access
Doctors have warned of the sweeping ramifications the ruling could have for patients.
Dr. Kristyn Brandi, an OB-GYN in New Jersey, told CBS News earlier this year that a ban on the drug would be “devastating.”
“A lot of people rely on this medication,” she said. “It is something that has been the standard of care for over 20 years.”
Democrats and pro-abortion rights groups lambasted the ruling from Kacsmaryk and reiterated their commitments to protecting abortion rights.
“This ruling from an activist judge is wildly out of step with the law and sets a dangerous new precedent,” Senate Majority Leader Chuck Schumer said in a statement. “Senate Democrats are relentlessly working to protect a woman’s right to choose from this extreme MAGA Republican agenda.”
Planned Parenthood president Alexis McGill Johnson called the decision an “outrage,” but noted that mifepristone can still be accessed for now.
“This decision could threaten the FDA’s role in this country’s public health system, and — if allowed to stand — will have broad and unprecedented consequences that reach far beyond abortion,” she said in a statement. “Let’s be clear: Those opposed to abortion are not satisfied with overturning Roe v. Wade and are actively seeking to erode access to sexual and reproductive health care by pursuing lawsuits like this one that undermine medical expertise and harm patients.”
The challenge to the abortion drug is the latest effort from anti-abortion rights advocates to limit access. In addition to abortion bans by gestational age and method, Republican-led states have also enacted laws limiting medication abortion.
Near-total abortion bans in 12 states supersede restrictions on medication abortion, while 15 states require medication abortion be provided by a physician. In six states, the patient must have an in-person visit with a medical practitioner, according to the Guttmacher Institute, a research group that supports abortion rights.
At the federal level, the Biden administration has taken steps to expand the availability of abortion pills. The FDA in January finalized a rule change that broadens availability of the drugs by allowing more retail pharmacies to dispense the drugs to patients with a prescription. Walgreens and CVS then said they intend to sell mifepristone.
The Justice Department’s Office of Legal Counsel also gave the green-light for the U.S. Postal Service to mail mifepristone and misoprostol, finding that the Comstock Act of 1873 does not prohibit the mailing of the drugs.
The case targeting mifepristone attracted significant public interest and scrutiny of Kacsmaryk, largely focusing on his conservative views and the motivation behind the suit being filed in Amarillo.
Critics of the anti-abortion rights groups have accused them of engaging in forum-shopping, a practice in which a party will pursue a claim in the court most likely to be favorable to them. Kacsmaryk also came under criticism after he delayed notifying the public of a hearing on March 15.
Read the full decision here:
[ad_2]
Source link